Title image above is attributed to Jynto, CC0, via Wikimedia Commons
(This article was originally published 8th February 2023 here on our Jujube Tree Nursery site.)
First published here 17th February 2023.
The Carbon Dioxide (CO2) Molecule
CO2 is a simple molecule of two oxygen atoms each double-bonded to one carbon atom.
It is such a simple molecule in fact, that it can even be written in text as O=C=O.
But of course these versions are far more impressive and sciency, yes?!
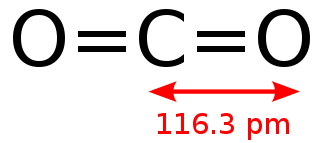
The Carbon dioxide (CO2) molecule
Attribution: Alessio Damato [Public domain]
3D ball model of the carbon dioxide (CO2) molecule
Attribution: Jynto, CC0, via Wikimedia Commons
Space-filling model of the carbon dioxide (CO2) molecule
Attribution: Jynto, CC0, via Wikimedia Commons
Chemists would describe this molecule as linear and centrosymmetric.
CO2 is also heavier than air!
Where Did CO2 Come From?
Early Earth is believed to have been a ball of molten rock (magma) when it formed around 4.5 billion years ago.
Through an absolutely amazing experiment where a miniature early Earth was simulated with that same, long-since solidified magma (rock), remelted — and levitated! — it would appear that Earth’s early atmosphere was 97% CO2 and 3% nitrogen gas (N2). The same as Venus’ and Mars’ to this day — not that surprising in hindsight really, considering the three planets formed at the same time and from similar materials.
The reason that, of the three, it was Earth’s atmosphere which changed with time is due to its larger size and particular distance from the sun. Venus was/is too hot, and Mars was/is too cold (and small), while Earth was/is ‘just right’ to be able to retain water on its surface. As Earth slowly cooled, water could condense on the surface and form an ocean.
More and more CO2 would dissolve into this growing ocean, and react to produce the weak acid carbonic acid (H2CO3). This molecule deprotonates (loses two protons, the two H+ ions) to hydrogencarbonate (also called bicarbonate, HCO3-) when in water. Hydrogencarbonate in turn deprotonates to the carbonate ion (CO32-), and all three molecules protonate and deprotonate to establish an equilibrium in water:
H2CO3 + 2H2O ⇌ HCO3- + H3O+ + H2O ⇌ CO32- + 2H3O+
Remember the carbonate ion (CO32-), as this will feature later!
Magma continually coming to the planet’s surface would bring with it dissolved gases which outgassed into the atmosphere as that magma cooled. These gases included still more CO2 and N2, water vapour (H2O), sulfur dioxide (SO2), hydrogen sulfide (H2S), methane (CH4), hydrogen (H2), carbon monoxide (CO), neon (Ne), helium (He) and Argon (Ar).
CO2 Concentrations Over Time
Today, atmospheric CO2 is but 0.04% of the atmosphere — quite the drop from 97%! From 970,000 parts per million (ppm) to just 400 ppm in 4.5 billion years.
From being the leading atmospheric gas by a country mile, to ranking a distant fourth after nitrogen (78%, or 780,840 ppm), oxygen (21%, or 209,460 ppm) and argon (0.93%, or 9,340 ppm)!
What happened? No one can definitively say, but we can still draw some plausible conclusions!
By 1.4 billion years ago, atmospheric CO2 concentration is believed to have dropped to 4,000-80,000 ppm — up to one-twelfth that originally, but still ten to two hundred times current levels.
Now the earliest known life dates from about 3.5 billion years ago, and however it arose here on Earth, whether from abiogenic origins or seeding by a meterorite(s), it was definitely microbial, and likely to have been autotrophic.
Autotrophs...fix carbon dioxide! They convert the carbon in CO2 into carbohydrates, which they then use to grow and reproduce. Some autotrophs further developed heterotrophy — as well as or instead of autotrophy — and consumed other autotrophs as their carbon source. This led to more organic carbon entering and circulating amongst these early food chains.
The first multicellular organisms appeared about 600 million years ago, and these had even greater carbon needs again. All fuelled, directly or indirectly, by carbon fixation of the atmosphere.
It makes too much sense for such a large drop in atmospheric CO2 to coincide with the rise of early life and increasing biomass, as more and more inorganic carbon became organic carbon in living organisms. (Similarly, the rise in atmospheric oxygen from next to nothing to 21% today is solely due to the appearance of cyanobacteria two billion years ago.)
Atmospheric CO2 was about 4,000 ppm during the Cambrian Period around 500 million years ago. This geological period is also known as the Cambrian Explosion, with many many multicellular organisms suddenly appearing in the fossil record. The first plants (multicellular autotrophs in other words!) appeared towards the end of this period.
Noteworthy too, was the rise of giant ammonites during this period. (Ammonites look like modern nautiluses but are actually more closely related to squids, octopuses and cuttlefish.) Fossil evidence suggests the shells of these animals could reach 2 m in diameter! And these shells were mostly calcium carbonate (CaCO3), a molecule made by the ammonites from calcium ions (Ca2+) and the carbonate ions (CO32-) mentioned above, which formed from dissolved CO2.
By about 60 million years ago, during the Cenozoic era when dinosaurs had gone and mammals, birds and flowering plants were dominant, atmospheric CO2 may still have been over 2,000 ppm. By 24 million years ago, as volcanic activity slowed, CO2 dropped more in line with today's levels, at 500 ppm.
CO2 and Life Today
There are seven known autotrophic pathways. Six do not use CO2 as their carbon source, are only found in microorganisms, and make up just 10% of all biological carbon fixing.
Which means the remaining 90% is by far the most dominant pathway, whereby CO2 is fixed via the Calvin Cycle. Plants utilise this pathway, and are the dominant carbon fixers on land. Algae and cyanobacteria also use this pathway, and are the dominant carbon fixers in water.
Photosynthesis shuts down in these dominant carbon fixers when CO2 levels drop below 150-200 ppm, yet growth can increase by up to 50% at 1,000 ppm.

Is CO2 plant food? Here is what happens with more CO2 molecule
Attribution: unknown.
Downloaded from https://qph.fs.quoracdn.net/main-qimg-16da9198ded4a58c57f302d55bf3561c
Commercial growers know this, and routinely pump CO2 into their greenhouses.
Is it possible that the CO2-fixing autotrophs are in a state of perpetual…starvation? How much more starved would they be if CO2 were not heavier than air?
Many people claim that talking to their plants makes them grow better. The concentration of CO2 in exhaled breath is about 4.4% — 44,000 ppm — and about 110 times that of ambient concentration.
Food (!) for thought.
































Leave a Comment